

Genetic or nutritional deficiencies that impede protein maturation are deleterious to health. In addition, during maturation posttranslational modifications may add new chemical groups or remove transiently needed peptide segments.

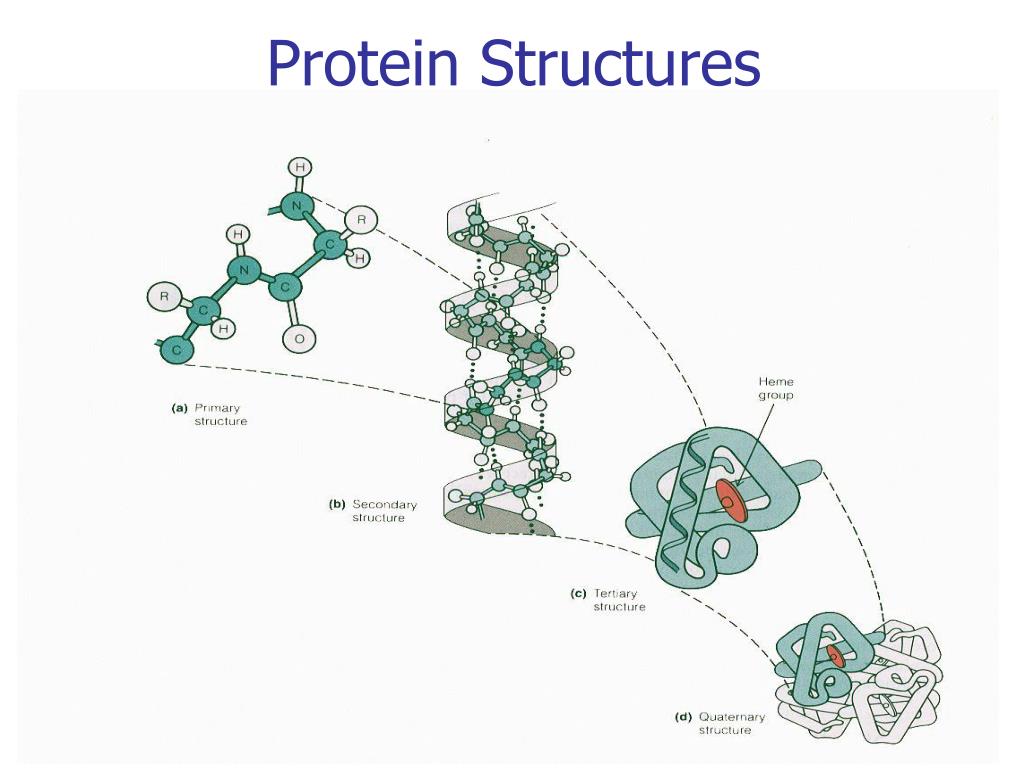
In order for a newly synthesized polypeptide to mature into a biologically functional protein capable of catalyzing a metabolic reaction, powering cellular motion, or forming the macromolecular rods and cables that provide structural integrity to hair, bones, tendons, and teeth, it must fold into a specific three-dimensional arrangement, or conformation. Identify the physiologic roles in protein maturation of chaperones, protein disulfide isomerase, and peptidylproline cis–trans isomerase.ĭescribe the principal biophysical techniques used to study tertiary and quaternary structure of proteins.Įxplain how genetic and nutritional disorders of collagen maturation illustrate the close linkage between protein structure and function.įor the prion diseases, outline the overall events in their molecular pathology and name the life forms each affects. Indicate the present state of knowledge concerning the stepwise process by which proteins are thought to attain their native conformation. Identify the major recognized types of secondary structure and explain supersecondary motifs.ĭescribe the kind and relative strengths of the forces that stabilize each order of protein structure.ĭescribe the information summarized by a Ramachandran plot. Indicate the advantages and drawbacks of several approaches to classifying proteins.Įxplain and illustrate the primary, secondary, tertiary, and quaternary structure of proteins. \) in the side chain of hydroxyproline allows more hydrogen bonding in the collagen peptide which makes connective tissues stronger.After studying this chapter, you should be able to:


 0 kommentar(er)
0 kommentar(er)
